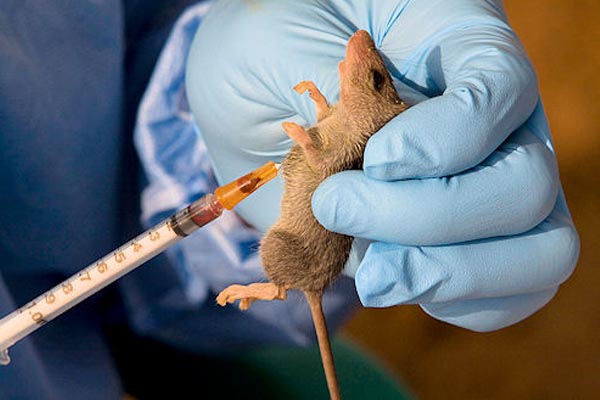
The global fight against Lassa fever has recorded another breakthrough as the Oxford Vaccine Group administered the first dose of a new Lassa fever vaccine to a volunteer in the United Kingdom.
The event, announced online on Thursday, took place in Oxford through the Oxford Vaccine Group, a unit of the University of Oxford’s Department of Paediatrics.
The milestone marks the start of a first-in-human clinical trial of the ChAdOx1 Lassa vaccine, funded by the Coalition for Epidemic Preparedness Innovations, CEPI.
Thirty-one healthy adults aged 18 to 55 will take part in the trial in Oxford, while a second phase-1 trial is expected to begin in Ghana early 2026.
Prof. Ramasamy Maheshi, Chief Investigator of the trial at Oxford, described the study as a “crucial step toward protecting vulnerable communities.”
“Vaccines are one of the most powerful tools we have in global health: they save lives, stop outbreaks, and strengthen health systems,” Maheshi said.
She noted that the vaccine was developed using the same platform as the Oxford/AstraZeneca COVID-19 vaccine, estimated to have saved six million lives in its first year alone.
“CEPI had earlier supported the preclinical development of the Lassa vaccine,” she added.
Dr Katrin Ramsauer, CEPI’s Lassa Disease Programme Lead, called the launch a “transformative milestone,” adding that progress so far reflects years of scientific innovation and global collaboration.
Ramsauer said that beyond the trial, regional efforts were underway to accelerate vaccine licensure and ensure equitable access.
“The Lassa Fever Coalition, led by the West African Health Organisation (WAHO) with support from CEPI and partners, is coordinating steps toward eventual rollout across affected countries,” she said.
According to Dr Virgil Lokossou, Director of Healthcare Services at WAHO, Lassa fever has affected lives in West Africa for more than half a century, impacting families, livelihoods, hospitals, and economies.
“Our region is now taking bold steps to change that story,” he said.
Public health authorities in Nigeria, including the Nigeria Centre for Disease Control and Prevention, NCDC, continue to record seasonal outbreaks, with healthcare workers among those at risk.
If successful, the Oxford vaccine could become one of the first licensed preventive vaccines against Lassa fever, a development experts say could dramatically reduce illness, deaths, and socioeconomic disruptions in West Africa.
Lassa fever, first discovered in Nigeria in the late 1960s, remains endemic across West Africa and continues to cause recurring outbreaks with high morbidity and mortality.
The virus, spread primarily by rodents, can lead to severe complications including deafness, bleeding, and death.
Source: NAN
READ ALSO:
BREAKING: Tinubu increases ambassador-nominees to 65, seeks Senate confirmation
NEC approves N100bn for rehabilitation of security agencies’ training institutions
The silent confusion about marriage certificates and travel
W’Africa ParaGames: Nigeria claim double gold
82 female Nigerians are on death row, says ASF France
Peoples parliament: Mixed verdicts at review of Tinubu administration
Senate confirms retired Gen Chris Musa as Defence Minister
EBA purchase: AMCON repays N3.6 trillion to CBN till date
Two die, nine injured in accident on Enugu-P’Harcourt Expressway